What are they?
Acidosis and alkalosis are terms used to describe abnormal conditions when a patient’s blood pH may not fall within the healthy range. Measuring the hydrogen ion concentration, and calculating the pH, of blood is a way of finding out how acidic or alkaline the blood is. Normal blood pH must be within a narrow range of 7.35-7.45 so that the body’s metabolic processes can work properly and can deliver the right amount of oxygen to tissues. Many diseases and other conditions can cause a patient’s blood pH to fall outside of these healthy limits.
In the human body, normal metabolism generates large quantities of acids (effectively compounds that produce a free hydrogen ion) that must be removed to keep a normal pH balance. Disruption of this balance can be caused by a build-up of acid or alkali (base) or by an increased loss of acid or alkali (see the diagram of ‘taps and drains’ below). Alkalis, or bases, are compounds that remove a free hydrogen ion. Acidosis occurs when blood pH falls below 7.35, indicating an increase in hydrogen ion concentration. Alkalosis occurs when blood pH rises above 7.45, indicating a reduction in hydrogen ion concentration. Both of these conditions act as an alarm to the body; they trigger actions intended to restore the balance and to return the blood pH to its normal range.
The major organs involved in regulating blood pH are the lungs and the kidneys. The lungs flush acid out of the body by exhaling carbon dioxide (CO2), which forms an acid when in solution (dissolved in the blood). Within physical limits, the body can raise and lower the rate of breathing to alter the amount of carbon dioxide that is breathed out. This can affect blood pH within seconds to minutes. The kidneys remove some acids in the urine and the kidneys also produce and regulate the retention of bicarbonate (HCO3-), a base (alkali) that increases the blood’s pH. Changes in bicarbonate concentration occur more slowly than changes in carbon dioxide, taking hours or days. Often, both of these processes proceed at the same time, continuing until the balance is restored or until the body’s ability to compensate is exhausted or overwhelmed. Diseases affecting either the lungs or the kidneys as well as other metabolic conditions can interfere with the regulation of blood pH.
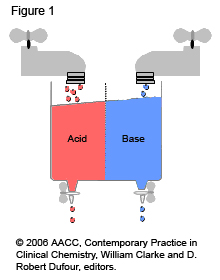
Figure 1: Taps and Drains
- The blood’s pH must remain between 7.35 and 7.45.
- The body tries to have a constant balance between incoming/produced acids and bases (tap on) and removal of acids and bases (drain open).
- Imbalances of the flow from the ‘taps’ and removal through the ‘drains’ lead to acidosis (acid sink overflow) or alkalosis (base sink overflow).
- Balance can be restored by increasing removal (faster flow through the ‘drain’) and/or by decreasing flow in (slowing the flow through the ‘tap’).
Acidosis or alkalosis can be an acute condition (develops quickly) or it may be a long-term chronic condition. Acidosis may not cause any symptoms or it may be associated with symptoms such as tiredness, feeling sick or vomiting. Acute acidosis may also cause an increased rate and depth of breathing, confusion, and headaches, and it can lead to fits, coma, and in some cases death. Symptoms of alkalosis are often due to potassium (K+) loss and may include irritability, weakness, and cramping.
Acid-base disorders are divided into two broad categories, respiratory and metabolic. Those that affect breathing and cause changes in carbon dioxide concentration are called respiratory acidosis (low pH) or respiratory alkalosis (high pH). Respiratory acid-base disorders are commonly due to lung diseases or conditions that affect normal breathing. Disorders that affect bicarbonate concentration are called metabolic acidosis (low pH) and metabolic alkalosis (high pH). Metabolic acid-base disorders may be due to kidney disease and a variety of other conditions. There are also known genetic abnormalities that result in the impairment of normal metabolic pathways and so can cause acid-base imbalance, usually acidosis. These are called inborn errors of metabolism (or genetic-metabolic disorders) and the acid-base effect is due to deficiencies or build-ups of compounds, many of which are acidic in nature. Other disorders that can cause metabolic (non-respiratory) acid-base disorders include diabetes (diabetic ketoacidosis), severe vomiting and severe diarrhoea.